A superior HEK cell line for AAV production
Writer’s Insight
Derived from ExcellGene’s HEKExpress® cells, a widely used cell system known for its transient and stable protein expression, a new host system with superior performance has been developed. This innovative system, cultivated in suspension with chemically defined media, offers remarkable enhancements.
A scientific paper, published by ExcellGene’s team of scientists in the prestigious journal of Biotechnology and Bioengineering titled: A new T-antigen negative HEK293 cell line with improved AAV productivity (Croissant et al. (2023)), unveils the development of a new HEK293-derived cell line that is devoid of all SV40 T-antigen sequences. This esteemed work was motivated by the rising safety concerns in the scientific community about the presence of the SV40 DNA sequences.
The resulting clonally derived HEKzeroT® cell line exhibits exceptional phenotypic characteristics in terms of robust growth, high transfectability, and the highest productivity of AAV virus-like particles.
Interested in our HEKzeroT® services? Make a request here.

Florian M. Wurm, Dr.
rer. nat., Prof. Emeritus
CSO, Co-Founder
Paper Details
Authors
Coralie Croissant | Joshua Armitano | Bertrand Lazuech | Daniel Švec | Cyril Pugin | Anaïs Guesdon | Louise Bryan | Antonio Castro | Léa Neuhaus | Giulia Fonti |
Jacopo Martinis | Maria J. Wurm | Florian M. Wurm | Paco Pino
Published In
Biotechnology and Bioengineering WILEY, 10970290, 2023, 0
doi.org/10.1002/bit.28414
ExcellGene SA, Monthey, Switzerland
Paper Abstract
Viral vectors for gene therapy, such as recombinant adeno-associated viruses, are produced in human embryonic kidney (HEK) 293 cells. However, the presence of the SV40 T-antigen-encoding CDS SV40GP6 and SV40GP7 in the HEK293T genome raises safety issues when these cells are used in manufacturing for clinical purposes. We developed a new T-antigen-negative HEK cell line from ExcellGene’s proprietary HEKExpress,® using the CRISPR- Cas9 strategy. We obtained a high number of clonally-derived cell populations and all of them were demonstrated T-antigen negative. Stability study a n dAAV production evaluation showed that the deletion of t h eT-antigen-encoding locus did not impact neither cell growth nor viability nor productivity. The resulting CMC-compliant cell line, named HEKzeroT,® is able to produce high AAV titers, from small to large scale.
KEYWORDS
AAV, gene therapy, HEK293, manufacturing, T-antigen

Coralie Croissant, PhD.
Research Associate

Paco Pino, PhD.
Director, Research and Development
1 | INTRODUCTION
Recombinant adeno-associated viruses (rAAV) have several advantages that make them vectors of choice for the development of gene therapy. They are nonpathogenic in humans, are able to infect dividing as well as nondividing cells, and different serotypes can target different specific organs/cells types (Sha et al., 2021; Wang et al., 2019). Today, the most common way to produce rAAV is by transient transfection of HEK (human embryonic kidney) 293 cells (Blessing et al., 2019; Sha et al., 2021; Strobel et al., 2019). In the 1970s, the HEK293 cell line was generated by transforming human primary embryonic kidney cells with viral DNA fragments from Adenovirus 5, resulting in the integration of E1A and E1B sequences into chromosome 19 (Lin et al., 2014; Louis et al., 1997; Russell et al., 1977). Ten years later, HEK293 were stably transfected with a plasmid carrying the coding sequences SV40GP6 and SV40GP7 encoding for the Simian virus (SV) 40 large and small T-antigens, respectively (DuBridge et al., 1987; Heinzel et al., 1988; Rio et al., 1985). The resulting new cell line, known as HEK293T, is commonly used in research and manufacturing. However, as reported in several studies, SV40 T-antigen and SV40-transformed cells are able to induce tumors in hamsters and mice (Eddy et al., 1962; Girardi et al., 1962; Reddel et al., 1993; Vilchez et al., 2003), which raises safety issue concerns when the production from HEK293T is intended for clinical use.
ExcellGene’s proprietary suspension-cultured HEKExpress® cell line in animal component-free media is derived from HEK293T cells. To reduce the previously-cited risks and provide safer rAAV of high quality, the T-antigen coding sequence was deleted from HEKExpress® genome, using the CRISPR-Cas9 strategy as previously described (Bae et al., 2020). Optimized transfection and cloning protocols lead to a fast, reliable, and CMC-compliant generation of a high number of clonally-derived cell populations. T-antigen screening revealed that 100% of the tested clones were T-antigen negative. Cell phenotypic parameters such as growth, viability, transfectability as well as AAV productivity were not affected by the deletion. On the contrary, AAV titers were even higher in some T-antigen-deleted clones than in the parental HEKExpress.® The resulting HEKzeroT® cell line, also maintained under suspension growth conditions in animal component-free media, sustains high growth rates and viabilities and generates high AAV titers from the mL-scale to the 10 s of liters.
2 | MATERIALS & METHODS
2.1 | Plasmid design
The gRNAs targeting the SV40 T-antigen coding sequence were synthetized and cloned into the pD1401-AD plasmid by ATUM. The gRNA sequences are as follow: gRNA_5′ (5′-TATGCTCATCAACCTGACTT-3′) and gRNA_3′ (5′-CAGCCATATCACATTTGTAG-3′), targeting the beginning and the end of the T-antigen coding sequence, respectively.
2.2 | Cell culture
The proprietary cell line HEKExpress® (ExcellGene) was used to generate, develop, and establish the clonally-derived HEK293T deleted for the T-antigen coding sequence.
Cells were grown in suspension at a cell density of 0.3−0.6 × 106 cells/mL in chemically-defined medium BalanCD HEK293 (Fujifilm) supplemented with GlutaMax™ from Gibco (Thermo Fisher Scientific) and shaken in a humidified ISF-1-W orbital shake incubator (Kuhner) at 140 rpm, 37°C, and 8% CO2.
2.3 | Single-cell cloning
Clonal cell lines were obtained by image-assisted cell distribution into single wells of 96-well plates (f.sight; Cytena GmbH). Wells containing single cells were identified using the QC images of single-cell dispenser device and image-based clonality assessment (Cell Metric®; Solentim). Obtained clones were expanded, transferred to TPP® TubeSpin 50 bioreactor tubes, and frozen in mini-banks.
2.4 | Generation of T-antigen-negative clonal populations
Optimized ExcellGene transfection and cloning media were used for the transfection and the culture of HEKExpress® cells. The transfection of cells was done in animal component-free medium using the vector pD1401-AD containing the gRNA sequences. Two days posttransfection, single-cell cloning was performed. Once clonal growth led to a well-established population, cells were transferred to suspension culture and further expanded.
2.5 | Clone screening
DNA of clones was extracted using the DNeasy Blood & Tissue Kit (Qiagen) according to manufacturer’s instructions. Clone screening was performed by duplex-qPCR (using two sets of primers). One primer set targets the large T antigen coding sequence (Fwd, 5′-TAAAGCATTGCCTGGAACGC-3′, Rev, 5′-AAACTCAGCCACAGGTCTGTAC-3′) and was used at a final concentration of 500 nM for each primer. The second primer set targets the human ACTB (β-Actin) coding sequence (Fwd, 5′-AACACGGCATTGTCACCAAC, Rev, 5′-TCTTTTCACGGTTGGCCTTG-3′) and was used at a final concentration of 100 nM for each primer. PCR reactions were performed using iTaq™ Universal SYBR® Green Supermix (BioRad) in two steps. After an initial denaturation at 95°C for 5 min, 39 cycles of denaturation (95°C, 15 s) and annealing-extension (60°C, 30 s) were performed. The PCR products from duplex-qPCR were diluted in 6× Gel Loading Purple Dye (New England BioLabs) and 5 or 10 µL were electrophoretically separated on 2% agarose gel pre-stained with SYBR™ Safe DNA Gel Stain (Thermo Fisher Scientific).
2.6 | Targeted locus amplification (TLA) analysis
TLA sequencing (de Vree et al., 2014) was performed by Cergentis using three sets of primers. Reads were aligned using the human HG38 genome as host reference genome sequence.
2.7 | AAV production
Triple transfections were performed on clonal populations for AAV production in different scale according to ExcellGene’s procedures and reagents: 10 mL-scale in bioreactor tubes; 25- and 250 mL-scales in shake flasks, and 1 L-scale in Mobius® 3L Single-use Bioreactor. Cells were cotransfected with a pHelper plasmid containing AAV helper genes pAAV-GFP (containing the GFP coding sequence flanked by AAV ITRs) and pRepCap8 or pRepCap9 (containing the AAV8 or AAV9 rep and cap genes, respectively). Cell cultures were harvested 72 h later, lysed with NP-40 (Merck KGaA) and treated with benzonase (Merck KGaA) for 1 h at 37°C. After addition of EDTA, samples were cleared either by centrifugation or filtration and supernatants were analyzed by AAV8 or AAV9 Xpress ELISA (both by Progen) and dPCR (QIAcuity by Qiagen) to determine AAV capsid titers and AAV genome titers, respectively. The primers and the probe used for dPCR target the GFP coding sequence (Fwd, 5′-TGCAAAGACCCCAACGAGAA-3′, Rev, 5′-GGCGGCGGTCACGAA-3′, probe, 5′-CGCGATCACATGGTCCTGCTGG-3′). Primers were used at a final concentration of 8 µM and the probe at a final concentration of 4 µM.
2.8 | Stability study (SS)
Cell populations were thawed from Research Cell Bank (RCB) and passaged every 3−4 days at a cell density of 0.3−0.6 × 106 cells/mL. For 2 months, cells were maintained in culture and banked every fourth passages (every other week) as SS-RCBs.
One vial per condition and SS-RCB were thawed, together with one vial from the initial RCB as a starting point. After recovery, cells were transfected and AAV8 titers were determined as described in the previous section.
3 | RESULTS AND DISCUSSION
3.1 | HEKExpress® genotypic characterization
To characterize the integration sites of the pRTAK plasmid carrying the T-antigen coding sequence originally integrated in the HEKExpress® cell line, TLA sequencing was performed. A single integration site of the pRTAK plasmid was found in the chromosome (chr) 3 equivalent, between positions 8,590,507 and 9,140,859 of the HG38 human reference genome sequence (Figure 1a,b), with a copy number estimated to be between 1 and 4. In addition, a genomic deletion of approximately 550 kb was detected between the identified junctions, represented by the green arrows in Figure 1b. According to the RefSeq, this region contained the genes CAV3, RAD18, and the exons 1 and 2 of SRGAP3. The Figure 1d represents the chr3 in red with this deletion and the integration of the plasmid carrying the T-antigen sequence, with the confirmed junctions in blue. These observations are consistent with a previous study where the authors characterized their HEK293T cell line (Bae et al., 2020).

Figure 1
Genotypic characterization of HEKExpress® cells. (a) TLA sequence coverage across HEKExpress® genome using the primers targeting the T-antigen coding sequence (set 1). The plot show that the integration site of the plasmid is located on the chromosome 3 equivalent (chr3). (b) TLA sequence coverage across the integration locus in human chr3 using the primers targeting the T-antigen coding sequence (set 1) or chr3 (set 3 and 4). The coverage of set 1 shows a deletion of 550 kb in HEKExpress® genome (green arrows) compared to the human hg38 genome. The coverage of sets 3 and 4 confirm the junctions of the integrated plamid pRTAK. (c) Map of pRTAK plasmid originally integrated in HEKExpress® cell line. The large and small T-antigen sequences are indicated in the dark purple and gRNAs (gRNA_beginning and gRNA_end) targeting the gene in orange. (d) Map of chr3 equivalent (in red) with the 550 kb deletion and the integration of the pRTAK plasmid containing the T-antigen sequence. Plasmid-chromosome junctions confirmed by TLA are indicated in blue. HEK, human embryonic kidney; TLA, targeted locus amplification.
3.2 | Generation of T-antigen-negative clonal populations
The CRISPR/Cas9 system was used to mediate T-antigen deletion in HEKExpress.® The design of gRNA sequences targeting the beginning and the end of the T-antigen coding sequence (Figure 1c) was based on the work of Bae et al. (2020). In this study, the authors cotransfected the cells with the plasmid carrying the CAS9 and gRNA sequences and cloned them by limiting dilution. Here, we improved the strategy using ExcellGene’s (proprietary) transfection and cloning media and an automating single-cell dispenser. As shown in Figure 2a, this device is able to recognize a single cell in the region of interest and dispense it into 96-well plates, together with quality control images to prove the monoclonality of the colonies. Two days after transfection of the plasmid encoding for both Cas9 and the gRNA sequences, 1823 single cells were dispensed (Figure 2b,c). Approximately 21% of the cells were able to form a monoclonally-derived cell population in 96-well plates, and 11.7% were expanded until banking (Figure 3b). The Figure 2b shows a single cell dispensed at D0 (day of single-cell cloning) and dividing until forming a colony at D13. From pool generation to clonal population banking, the whole procedure was achieved within a month. Compared to the limiting dilution method that is traditionally used for single-cell isolation in numerous studies (Bae et al., 2020; Levin et al., 2020; Spidel et al., 2016; Thorne et al., 2009; Yuan et al., 2011), our procedure does not only enable the high-throughput isolation of cells in a short period of time, but also provides the required traceability to work in a CMC-compliant environment.
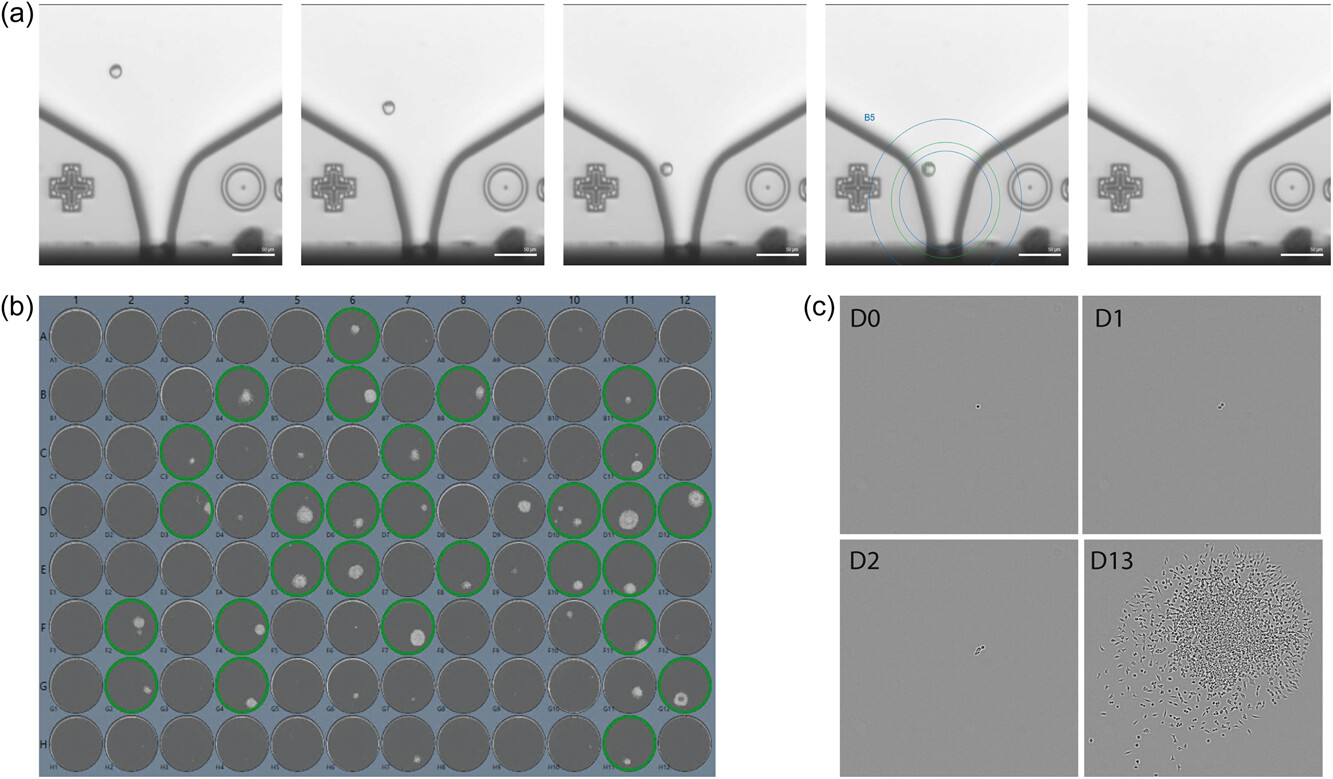
Figure 2
CMC compliant cloning of HEKEpress® cells after CRISPR/Cas9. (a) Images from the single-cell dispenser showing a single cell to dispense. The first four images show the cell coming in the ROI (blue circle). The last image is taken after the dispensing and show that the cell was successfully dispensed. Scale bar: 50 µm. (b) Scan of a 96-well plate at Day 13 post-seeding. Wells surrounded in green indicate monoclonally-derived cell populations ready to be transferred for expansion and represent approximatively 21% of the total printed cells. (c) Images from the clone imager device taken at D0 (Day 0), D1, D2, and D13 after single-cell cloning. The image at D0 confirms the dispensing of a single cell, that divides into two cells at D1 until forming a single-cell derived colony at D13. Scale bar: 200 µm. HEK, human embryonic kidney; ROI, region of interest.

Figure 3
Analysis of T-antigen removal on clonally derived cell populations. (a) A duplex-qPCR was performed on genomic DNA extracts, using two sets of primers targeting the T-antigen and the ß-ACTIN coding sequences. PCR products (5 or 10 µL) were then electrophoretically separated on agarose gel. The PCR products sizes from the T-antigen and ß-ACTIN targeting primers pairs are 107 and 141 bp, respectively. (b) Summary of single-cell cloning and KO colonies.
3.3 | Analysis of T-antigen-negative clonal populations
Several approaches were used to assess the T-antigen-encoding CDS deletion in clonal populations. First, a simple qPCR was performed. All tested clonal populations were lacking the T-antigen sequence (data not shown). To confirm that the absence of PCR product is due to the deletion, a duplex-qPCR was performed using two sets of primers, one targeting the T-antigen coding sequence SV40GP6, and another targeting β-ACTIN. The PCR products were subjected to agarose-electrophoresis (Figure 3a). The amplification from the SV40GP6 and β-ACTIN coding sequences should lead to the presence of a 107 bp fragment and a 141 bp fragment, respectively. As illustrated in Figure 3a, the β-ACTIN amplification product was present in the profile of both HEKExpress® and clones, showing that the PCR reactions occurred and the β-ACTIN sequence was, as expected, present in the DNA extracted from the tested cells. On the contrary, the T-antigen product was present in HEKExpress® parental cells but not in the tested clones, demonstrating that the T-antigen coding sequence is absent in the clones. Altogether, our results showed that the locus encoding for the small and large T antigens was successfully deleted in 100% of the screened clones (Figure 3b), while this percentage reached only 33% in the work of Bae et al. (2020). Our strategy combining improved transfection and cloning protocols thus leads to a faster yet reliable workflow for monoclonally-derived cell line generation.
3.4 | AAV productivity screening
AAV8 productivity was then assessed to screen the clonal populations. Cells were transfected with the triple-transfection method and harvested 72 h posttransfection. The HEKExpress® cell line was also included as a reference. As shown in Figure 4, AAV8 titers varied from a clonal population to another, from 67% to 254% for capsid titers and from 13% to 203% in genome titers, when compared to titers in HEKExpress.® This suggests that the T-antigen deletion itself does not impact AAV production and that highly productive clones have been isolated. Based on this data and on other parameters (transfection efficiency, productivity, and full/empty capsid ratio, data not shown), five top clones were selected for further analysis: #6, #8, #23, #38, and #114.
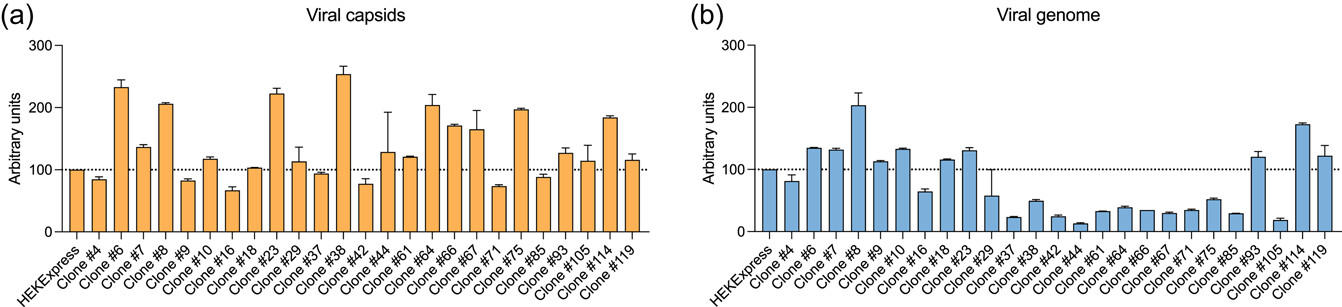
Figure 4
AAV productivity of T-antigen-negative clonally derived cell populations. Triple transfection was performed on cells and AAVs were harvested 72 h later. Viral capsid (a) and genome (b) titers were determined by ELISA and dPCR, respectively. Data were normalized with titers from HEKExpress® as reference. Error bars represent means ± standard deviation of two technical replicates. AAV, adeno-associated viruses; HEK, human embryonic kidney.
3.5 | Stability study
The five top clones were evaluated during a SS in which cell parameters and AAV production were assessed. Along with HEKExpress® as reference, cells were maintained in culture for 2 months with regular generation of SS-banks every other week. HEKExpress® as well as the clonal populations showed a constant high viability and high viable cell densities (VCD) up to 10−15 × 106 cells/mL (Figure 5a). Furthermore, the doubling time was stable and similar between the parental cell line and the clones, with an average of 20 h (Figure 5b). This evidence demonstrates that the T-antigen deletion does not affect cell growth, in contrast with previous observations by Bae et al. (2020) who noticed that their T-antigen clones were growing slower, probably due to other, unidentified factors. One vial of each SS-bank was then thawed to perform triple transfection for AAV8 production. From P00 (passage 00 corresponding to the initial bank) to P16 (passage 16, 2 months later), AAV8 produced in HEKExpress® had similar titers, with approximately 6 × 1011 viral capsids/mL and 8 × 1010 viral genomes copies/mL, showing that AAV production is stable even after 50−60 generations (Figure 5c,d). On the contrary, clones #6 and #38 presented variations in titers after several passages. AAV production in clone #6 decreased with the time, from 1 × 1011 to 1 × 108 viral capsids/mL, and from 1 × 1010 to 1 × 108 viral genome copies/mL. It is of importance to note that a copy number of 108 is the limit of detection for both ELISA and dPCR, meaning that the lowest measured titers are not accurate and might be overestimated. Clone #38 was unable to produce AAV at P00 and titers at other passages show variation of one to two logs. These data indicate that these clones are not stable for AAV production and were therefore not chosen for further investigation. Clones #8, #23, and #114 behaviors are similar to the parental line HEKExpress®: stable production of viral capsids and genome up to 1 × 1012 copies/mL and 3 × 1011 copies/mL, respectively. These AAV titers, observed in HEKExpress® and T-antigen negative cells, are in line with titers obtained with other HEK293 cell lines in the literature (Chahal et al., 2014; Grieger et al., 2016; Vandenberghe et al., 2010; Zhao et al., 2020).
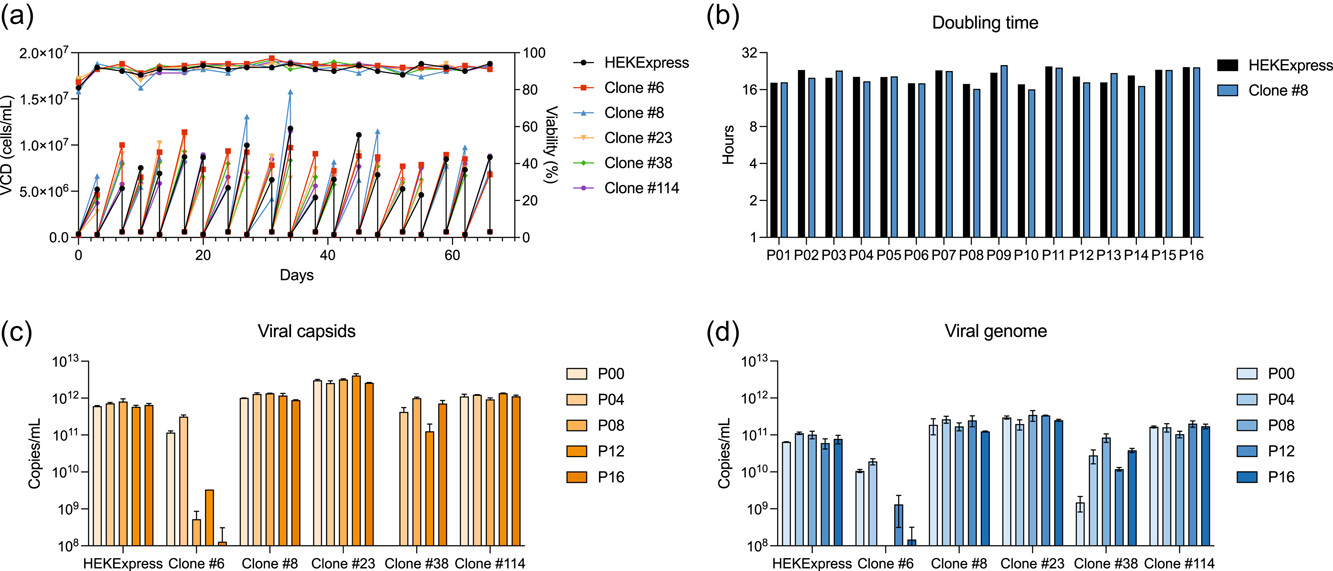
Figure 5
T-antigen negative cell performance and stability. Cells were maintained in culture for 2 months, with regular banking every other week. “PXX” represents the passage number of the cell culture after a bank was generated. Viable cell densities and viabilities were measured (a) and doubling times calculated (b). The data corresponding to clone #8 in (b) are representative of other top clones. (c, d) One vial of each bank was thawed and AAV8 virions were produced as described in Figure 4. Viral capsid (c) and genome (d) titers were determined by ELISA and dPCR, respectively. Error bars represent means ± standard deviation of two technical replicates. AAV, adeno-associated viruses.
Taking into account the data obtained from AAV production screening and SS, the clone #8 was chosen to go on with a scale-up process.
3.6 | Scalability study
Scalability of AAV production in top clone #8 was then assessed. Cells were seeded at 2 × 106 cells/mL and transfected for AAV9 production in 10 mL-, 25 mL-, 250 mL,- and 1 L-scale, respectively. Cell parameters were measured every day and harvest was performed at Day 3 posttransfection. AAV9 capsid and genome titers were determined by ELISA and dPCR. As shown in Figure 6a, cell parameters are very similar between the different conditions, with a maximum VCD at D3 between 5.5 × 106 and 6.4 × 106 cells/mL and viability above 90%. When grown in 1 L stirred tank reactor, VCD and viability are slightly lower than in smaller scales (e.g., a viability of 83% was observed at D3). Interestingly, this small difference does not have an impact on AAV9 production, since no apparent difference was observed for capsid as well as for genome titers from 10 mL- to 1 L-scale production (Figure 6b). Thus, clone #8 is able to produce high AAV titers in a stable way at different scales and was chosen for the establishment of the HEKzeroT® cell line.

Figure 6
T-antigen negative cell performance in different scale bioreactors. Clone #8 was seeded in different working volumes from 10 mL to 1 L and transfected for AAV9 production as described in Figure 4. (a) Viable cell density (VCD, left y-axis) and viability (line graphs right y-axis) were assessed every day. (b) Viral capsid and genome titers were determined by ELISA and dPCR, respectively. AAV, adeno-associated viruses.
4 | CONCLUSIONS
T-antigen negative monoclonally-derived cell populations were successfully and quickly generated thanks to optimized ExcellGene’s proprietary transfection and cloning protocols and in a CMC-compliant environment. The final clone chosen as HEKzeroT® showed higher AAV titers than the parental cell line HEKExpress® without any disturbance in cell growth, stability, and scalable AAV production. Altogether, this makes HEKzeroT® cell line a preferred choice for the safe and high-throughput manufacturing of rAAV aimed at the development of new gene therapies.
AUTHOR CONTRIBUTIONS
Acquisition and analysis of data: Coralie Croissant, Joshua Armitano, Bertrand Lazuech, Danijel Švecanijel ŠvecS, Cyril Pugin, Anaïs Guesdon, Louise Bryan, Antonio Castro, Léa Neuhaus, Giulia Fonti, and Jacopo Martinis. Study design, interpretation of data and manuscript drafting: Coralie Croissant, Joshua Armitano, Maria J. Wurm, Florian M. Wurm, and Paco Pino.
ACKNOWLEDGMENTS
We thank the reviewers for their useful comment and careful review.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
We hope you enjoyed this scientific paper from ExcellGene’s team of scientists. If you’d like to learn more about our company and the services we offer, please contact us here.
References
, , , , , , , , , , & (2020). Design and testing of vector-producing HEK293T cells bearing a genomic deletion of the SV40 T antigen coding region. Molecular Therapy-Methods & Clinical Development, 18, 631– 638. https://doi.org/10.1016/j.omtm.2020.07.006
, , , , , , , & (2019). Scalable production of AAV vectors in orbitally shaken HEK293 cells. Molecular Therapy-Methods & Clinical Development, 13, 14– 26. https://doi.org/10.1016/j.omtm.2018.11.004
, , , , & (2014). Production of adeno-associated virus (AAV) serotypes by transient transfection of HEK293 cell suspension cultures for gene delivery. Journal of Virological Methods, 196, 163– 173. https://doi.org/10.1016/j.jviromet.2013.10.038
, , , , , & (1987). Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Molecular and Cellular Biology, 7(1), 379– 387. https://doi.org/10.1128/mcb.7.1.379-387.1987
, , , & (1962). Identification of the oncogenic substance in rhesus monkey kidney cell cultures as simian virus 40. Virology, 17, 65– 75. https://doi.org/10.1016/0042-6822(62)90082-x
, , , & (1962). Development of tumors in hamsters inoculated in the neonatal period with vacuolating virus, SV-40. Experimental Biology and Medicine, 109, 649– 660. https://doi.org/10.3181/00379727-109-27298
, , & (2016). Production of recombinant adeno-associated virus vectors using suspension HEK293 cells and continuous harvest of vector from the culture media for GMP FIX and FLT1 clinical vector. Molecular Therapy, 24(2), 287– 297. https://doi.org/10.1038/mt.2015.187
, , , & (1988). Use of simian virus 40 replication to amplify Epstein-Barr virus shuttle vectors in human cells. Journal of Virology, 62(10), 3738– 3746. https://doi.org/10.1128/JVI.62.10.3738-3746.1988
, , , , & (2020). Production, purification and characterization of recombinant human R-spondin1 (RSPO1) protein stably expressed in human HEK293 cells. BMC Biotechnology, 20(1), 5. https://doi.org/10.1186/s12896-020-0600-0
, , , , , , , , , , , , , , , & (2014). Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations. Nature Communications, 5, 4767. https://doi.org/10.1038/ncomms5767
, , & (1997). Cloning and sequencing of the cellular-viral junctions from the human adenovirus type 5 transformed 293 cell line. Virology, 233(2), 423– 429. https://doi.org/10.1006/viro.1997.8597
, , , , , , , & (1993). Development of tumorigenicity in simian virus 40-immortalized human bronchial epithelial cell lines. Cancer Research, 53(5), 985– 991. https://www.ncbi.nlm.nih.gov/pubmed/8094998
, , & (1985). A mammalian host-vector system that regulates expression and amplification of transfected genes by temperature induction. Science, 227(4682), 23– 28. https://doi.org/10.1126/science.2981116
, , , & (1977). Characteristics of a human cell line transformed by DNA from human adenovirus type 5. Journal of General Virology, 36(1), 59– 72. https://doi.org/10.1099/0022-1317-36-1-59
, , , , , , , , , , & (2021). Cellular pathways of recombinant adeno-associated virus production for gene therapy. Biotechnology Advances, 49, 107764. https://doi.org/10.1016/j.biotechadv.2021.107764
, , , , & (2016). Rapid high-throughput cloning and stable expression of antibodies in HEK293 cells. Journal of Immunological Methods, 439, 50– 58. https://doi.org/10.1016/j.jim.2016.09.007
, , , , , , , & (2019). Standardized, scalable, and timely flexible adeno-associated virus vector production using frozen high-density HEK-293 cell stocks and CELLdiscs. Human Gene Therapy Methods, 30(1), 23– 33. https://doi.org/10.1089/hgtb.2018.228
, , & (2009). Manufacturing recombinant adeno-associated viral vectors from producer cell clones. Human Gene Therapy, 20(7), 707– 714. https://doi.org/10.1089/hum.2009.070
, , , , , & (2010). Efficient serotype-dependent release of functional vector into the culture medium during adeno-associated virus manufacturing. Human Gene Therapy, 21(10), 1251– 1257. https://doi.org/10.1089/hum.2010.107
, , , , & (2003). Simian virus 40 in human cancers. The American Journal of Medicine, 114(8), 675– 684. https://doi.org/10.1016/s0002-9343(03)00087-1
, , , , , , , , , , , , , , , , , , , … (2014). Targeted sequencing by proximity ligation for comprehensive variant detection and local haplotyping. Nature Biotechnology, 32(10), 1019– 1025. https://doi.org/10.1038/nbt.2959
, , & (2019). Adeno-associated virus vector as a platform for gene therapy delivery. Nature Reviews Drug Discovery, 18(5), 358– 378. https://doi.org/10.1038/s41573-019-0012-9
, , , , & (2011). A versatile adeno-associated virus vector producer cell line method for scalable vector production of different serotypes. Human Gene Therapy, 22(5), 613– 624. https://doi.org/10.1089/hum.2010.241
, , , , , , , , & (2020). Creation of a high-yield AAV vector production platform in suspension cells using a design-of-experiment approach. Molecular Therapy-Methods & Clinical Development, 18, 312– 320. https://doi.org/10.1016/j.omtm.2020.06.004